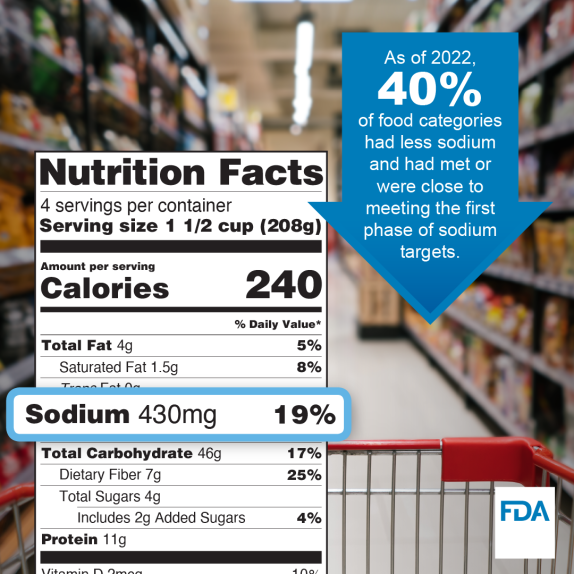
The U.S. Food and Drug Administration marked a milestone building on Phase I of its voluntary sodium reduction targets recently and issued draft guidance for Phase II in a data-driven, stepwise approach to help sodium reduction across the food supply. Prior to 2021, consumer intake was approximately 3,400 milligrams per day on average, far higher than the limit recommended by the Dietary Guidelines for Americans of 2,300 milligrams per day for those 14 years and older. If finalized, the new set of voluntary targets would support reducing average individual sodium intake to about 2,750 milligrams per day. This reduction is approximately 20% lower than consumer intake levels prior to 2021.
The Phase II voluntary sodium reduction targets follow an initial set of targets issued in October 2021. The initial set of targets encouraged the food industry to reduce sodium levels in a wide variety of processed, packaged, and prepared foods. Preliminary data from 2022 show about 40% of the initial Phase I targets are very close to or have already been reached indicating early success of this effort.
“Reducing sodium in the food supply has the potential to be one of the most important public health initiatives in a generation. The early successes we’re seeing with sodium level reduction in certain foods is encouraging and indicative of the impact we believe our overall nutrition approach can have on the wellbeing of society,” said FDA Deputy Commissioner for Human Foods Jim Jones. “In addition to our sodium reduction efforts, the FDA is also actively working on a forthcoming final rule updating the definition of the claim ‘healthy,’ a proposed rule for front-of-package nutrition labeling and exploring ways to reduce added sugars consumption. The FDA’s sodium reduction and other nutrition initiatives are central to a broader, whole-of-government approach to help reduce the burden of diet-related chronic diseases and advance health equity.”
The Phase II targets will continue to focus on commercially processed, packaged, and prepared foods in the marketplace. This guidance is particularly relevant as more than 70% of sodium intake in the U.S. population comes from sodium added during food manufacturing and commercial food preparation. The preliminary Phase I data released today, along with public and external feedback, informed the draft Phase II targets.
The U.S. faces an ever-growing epidemic of diet-related chronic diseases. Too much sodium can raise blood pressure, a major risk factor for heart disease and stroke. Strong scientific evidence supports lowering sodium intake from current levels. Reducing sodium intake has the potential to prevent hundreds of thousands of premature deaths and illnesses in the coming years by helping to reduce risk for heart disease and stroke. Because underserved communities, including racial and ethnic minority groups, experience high blood pressure at increased rates compared to the overall average, reducing sodium in the food supply also could help advance health equity for these populations.
The agency’s sodium reduction initiative is part of the White House National Strategy on Hunger, Nutrition and Health to reduce diet-related diseases by 2030. The FDA’s Phase II voluntary sodium reduction targets reflect what is known about achievable reductions in different food categories, consumer acceptance, and food safety, and align with the Healthy People 2030 goal of reducing average individual sodium intake to approximately 2,750 milligrams per day in the U.S. The Phase II voluntary sodium reduction targets also work in concert with the U.S. Department of Agriculture’s school meals sodium limits, so children have access to healthy choices inside and outside of school.
Additional sodium-related actions the FDA has taken include: the issuance of a proposed rule to amend the standards of identity to permit the use of salt substitutes in foods for which salt is a required or optional ingredient, and guidance on use of the term “potassium salt” instead of “potassium chloride” to signal consumers that the ingredient is a salt substitute.
The FDA will continue its stepwise approach to sodium reduction. The agency will also issue a complete evaluation of industry’s progress against the Phase I targets when the data from 2024 become available and are analyzed. The FDA expects to issue regular evaluations of sodium levels in foods about every three years to support its science-driven, transparent, and stepwise process. Future phases of sodium reduction targets will be considered as part of the agency’s evaluation and monitoring of sodium reduction progress in the marketplace, as well as monitoring of sodium intake in the population.

There are no comments
Please login to post comments